Insulin is a vital peptide hormone produced by the pancreas that regulates glucose metabolism in the body. First identified in the early 20th century, it revolutionized the treatment of diabetes mellitus, a metabolic disease characterized by impaired insulin production or action. This article provides a comprehensive overview of insulin, including its structure, physiological roles, history, synthesis, and therapeutic applications, as well as emerging trends in research.
Table of Contents
ToggleStructure and Synthesis of Insulin
Insulin is a protein hormone composed of two peptide chains, labeled A and B, connected by disulfide bonds. The A-chain contains 21 amino acids, while the B-chain contains 30 amino acids, resulting in a total molecular weight of approximately 5800 Daltons. These chains are linked through two inter-chain disulfide bridges, and there is an additional intra-chain disulfide bridge within the A-chain.
The structure of insulin is highly conserved across species, with only slight variations in amino acid sequences. For instance, porcine (pig) insulin differs from human insulin by only one amino acid, which made it a close substitute for therapeutic purposes before recombinant DNA technology enabled the production of human insulin.
Synthesis of Insulin
Insulin is synthesized in the beta cells of the islets of Langerhans in the pancreas. The synthesis occurs through a multi-step process:
- Preproinsulin Formation
- Conversion to Proinsulin
- Formation of Mature Insulin
- Storage and Secretion
Preproinsulin Formation
Translation of the INS gene on chromosome 11 produces preproinsulin, a single polypeptide precursor consisting of a signal peptide, the A-chain, B-chain, and a connecting peptide (C-peptide).
Conversion to Proinsulin
The signal peptide is cleaved in the endoplasmic reticulum (ER), forming proinsulin, which folds into its characteristic three-dimensional structure with the help of disulfide bonds.
Formation of Mature Insulin
Proinsulin is transported to the Golgi apparatus, where enzymes cleave the C-peptide, resulting in mature insulin and free C-peptide.
Storage and Secretion
Insulin is stored in secretory granules within the beta cells and released into the bloodstream in response to elevated glucose levels.
The measurement of C-peptide levels in the blood is used clinically to assess endogenous insulin production, as C-peptide and insulin are secreted in equimolar amounts.
Regulation of Insulin Secretion
Role of Blood Glucose
The primary stimulus for insulin secretion is an increase in blood glucose levels. The process involves several steps:
- Glucose enters beta cells through GLUT2 transporters.
- Inside the cell, glucose is phosphorylated and metabolized to produce ATP.
- Elevated ATP/ADP ratios close ATP-sensitive potassium (K+) channels, depolarizing the cell membrane.
- Depolarization triggers the opening of voltage-gated calcium (Ca2+) channels.
- Increased intracellular calcium stimulates exocytosis of insulin-containing granules.
Other Regulators
In addition to glucose, insulin secretion is modulated by:
- Amino Acids – Certain amino acids (e.g., leucine, arginine) stimulate insulin release.
- Incretin Hormones – Gut-derived hormones like glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) enhance insulin secretion in response to food intake.
- Autonomic Nervous System – Parasympathetic stimulation promotes insulin release, while sympathetic stimulation inhibits it.
- Hormones – Hormones like glucagon and growth hormone influence insulin release.
Physiological Role of Insulin
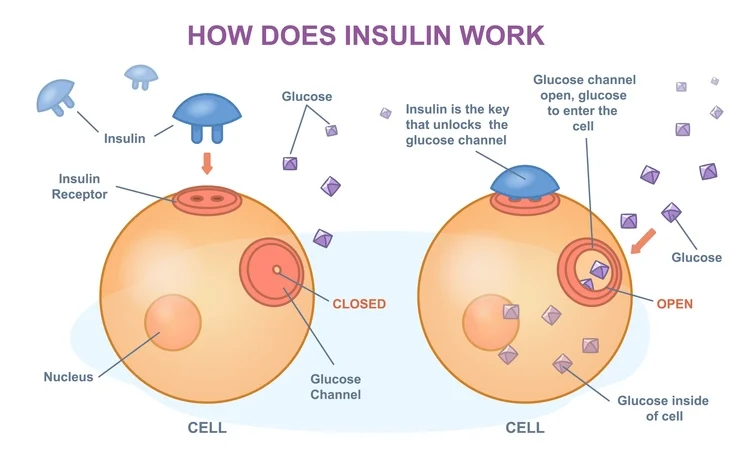
Insulin is the primary hormone responsible for lowering blood glucose levels. Its effects are mediated through binding to insulin receptors on target cells, leading to a cascade of intracellular events that promote glucose uptake, utilization, and storage. Key physiological roles of insulin include:
- Glucose Uptake and Metabolism
- Regulation of Fat Metabolism
- Protein Metabolism
- Inhibition of Gluconeogenesis
Glucose Uptake and Metabolism
Insulin facilitates glucose uptake into cells, particularly in muscle, adipose, and liver tissues, by promoting the translocation of glucose transporters (GLUT4) to the cell membrane. Once inside the cell, glucose is metabolized through glycolysis or stored as glycogen (glycogenesis).
Regulation of Fat Metabolism
Insulin promotes lipid synthesis (lipogenesis) in adipose tissue and inhibits lipolysis, thereby preventing excessive breakdown of fats.
It increases triglyceride storage by stimulating the uptake of fatty acids and glycerol into adipocytes.
Protein Metabolism
Insulin promotes protein synthesis and inhibits protein degradation. It stimulates the uptake of amino acids into muscle cells, thereby facilitating muscle growth and repair.
Inhibition of Gluconeogenesis
In the liver, insulin suppresses gluconeogenesis (the production of glucose from non-carbohydrate sources) and glycogenolysis (the breakdown of glycogen to glucose).
History of Insulin Discovery
The discovery of insulin marked a turning point in the treatment of diabetes mellitus.
- 1869: Paul Langerhans identified the pancreatic islets, later named the islets of Langerhans.
- 1889: Oskar Minkowski and Joseph von Mering demonstrated that removing the pancreas in dogs caused diabetes.
- 1921: Frederick Banting and Charles Best successfully isolated insulin from the pancreas of dogs under the supervision of John Macleod. James Collip purified the extract for therapeutic use.
- 1922: The first patient, Leonard Thompson, received insulin treatment and showed dramatic improvement.
- 1923: Banting and Macleod received the Nobel Prize for Medicine for their work on insulin.
Over the decades, advances such as recombinant DNA technology in the 1970s enabled the production of human insulin, which improved safety and efficacy for patients.
Insulin Deficiency and Diabetes Mellitus
Types of Diabetes
- Type 1 Diabetes (T1D)
- Type 2 Diabetes (T2D)
- Gestational Diabetes
Type 1 Diabetes (T1D)
An autoimmune condition characterized by the destruction of beta cells, resulting in little or no insulin production.
Type 2 Diabetes (T2D)
A metabolic disorder involving insulin resistance and relative insulin deficiency.
Gestational Diabetes
Diabetes occurring during pregnancy due to hormonal changes that affect insulin sensitivity.
Effects of Insulin Deficiency
Without sufficient insulin, glucose cannot enter cells efficiently, leading to hyperglycemia. Chronic hyperglycemia causes:
- Polyuria (excessive urination)
- Polydipsia (excessive thirst)
- Polyphagia (excessive hunger)
- Weight loss, fatigue, and blurred vision
Long-term complications include:
- Microvascular Damage: Retinopathy, nephropathy, and neuropathy
- Macrovascular Disease: Cardiovascular disease and stroke
- Ketoacidosis: In type 1 diabetes, severe insulin deficiency leads to the accumulation of ketone bodies, resulting in diabetic ketoacidosis (DKA), a life threatening condition.
Therapeutic Use of Insulin
Insulin formulations are categorized based on their onset, peak, and duration of action:
- Rapid-Acting Insulin: Lispro, Aspart, Glulisine
- Short-Acting Insulin: Regular human insulin
- Intermediate-Acting Insulin: NPH insulin
- Long-Acting Insulin: Glargine, Detemir, Degludec
- Pre-Mixed Insulin: Combinations of short-acting and intermediate-acting insulins
Insulin Delivery Methods
- Syringes and Vials: Traditional method of insulin administration
- Insulin Pens: More convenient and accurate dosing
- Insulin Pumps: Continuous subcutaneous insulin infusion (CSII) for better glucose control
- Inhaled Insulin: Alternative for those who dislike injections
- Artificial Pancreas: Closed-loop systems that automate insulin delivery based on glucose levels
Advances in Insulin Research
- Smart Insulins
- Gene Therapy
- Nanotechnology
Smart Insulins
Researchers are developing glucose-responsive insulins that release insulin only when blood glucose levels are elevated.
Gene Therapy
Gene therapy aims to regenerate beta cells or deliver insulin genes to non-beta cells, enabling endogenous insulin production.
Nanotechnology
Nanotechnology is being explored to improve insulin delivery through targeted drug delivery systems, enhancing bioavailability and reducing side effects.
Frequently Asked Questions (FAQs)
What is insulin resistance?
Insulin resistance occurs when the body’s cells do not respond properly to insulin, leading to increased blood glucose levels. It is a key feature of Type 2 Diabetes and metabolic syndrome.
How is insulin administered?
Insulin is commonly administered via subcutaneous injection using syringes, insulin pens, or insulin pumps. Some newer forms include inhaled insulin.
Can insulin be taken orally?
Currently, insulin cannot be taken orally because it is a protein that would be broken down by digestive enzymes before reaching the bloodstream. Research is ongoing into oral insulin formulations.
What are the symptoms of low insulin levels?
Low insulin levels can lead to hyperglycemia, characterized by symptoms such as excessive thirst, frequent urination, fatigue, and blurred vision. In severe cases, it can cause diabetic ketoacidosis (DKA).
Are there side effects of insulin therapy?
Yes, insulin therapy can cause side effects, including hypoglycemia (low blood sugar), weight gain, and injection site reactions. Proper monitoring and dose adjustments help mitigate these effects.
Can lifestyle changes reduce insulin dependency?
In Type 2 Diabetes, lifestyle changes such as a healthy diet, regular exercise, and weight management can improve insulin sensitivity and, in some cases, reduce or eliminate the need for insulin therapy.
Conclusion
Insulin is a critical hormone that plays a central role in maintaining glucose homeostasis. Its discovery and therapeutic application have transformed the management of diabetes mellitus, saving millions of lives worldwide. Advances in insulin therapies, including smart insulins, gene therapy, and artificial pancreas systems, hold great promise for improving diabetes care. As research progresses, the goal of achieving near-physiological glucose control for all patients with diabetes becomes increasingly attainable.